MEDICAL DEVICE AND ITS BIOCOMPATIBILITY TESTING REQUIREMENT TABLE AS PER FDA 510 (K) G95-MEMORANDUM AND ISO 10993-1
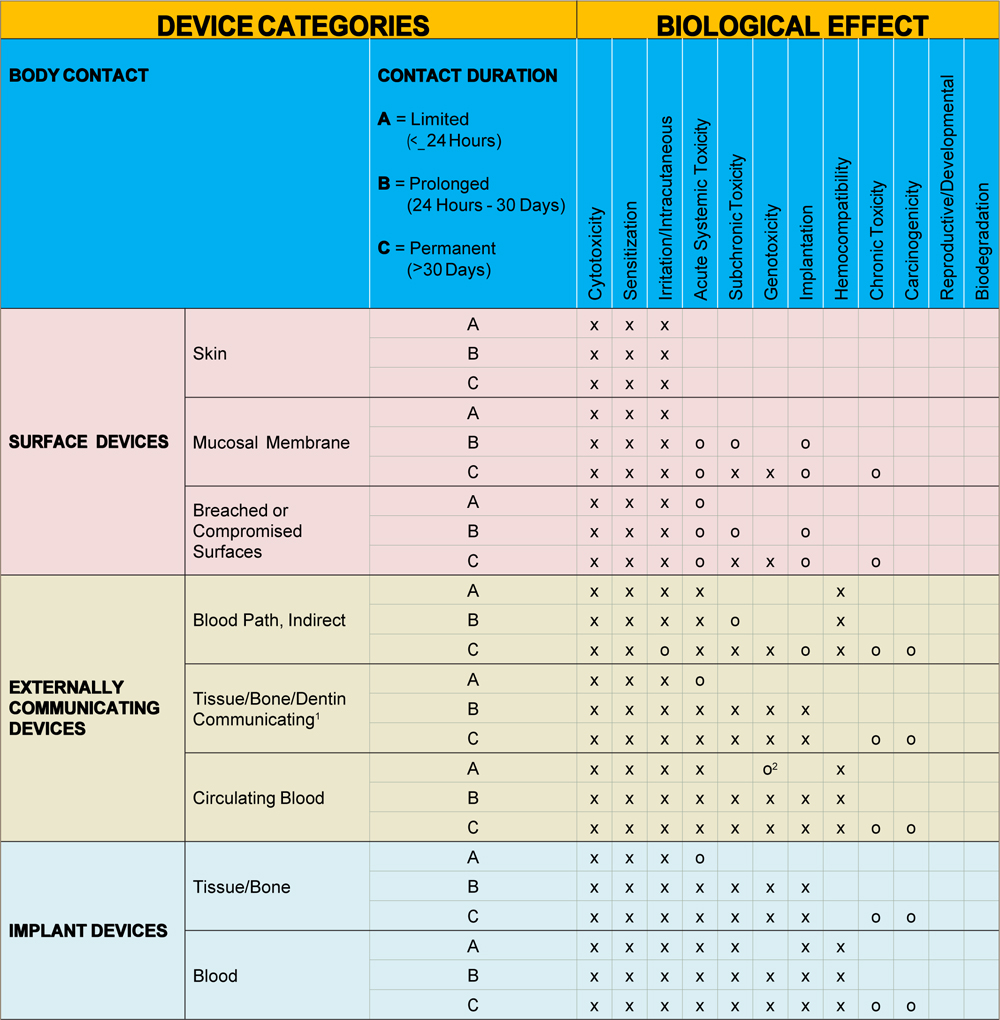
X = Tests per ISO 10993-1
O = Additional tests that may be applicable in the U.S.
Note1 – Tissue includes tissue fluid and subcutaneous spaces
Note2 – For all devices used in extracorporeal circuits




